by James Lyons-Weiler, PhD, Popular Rationalism, ©2024
(Apr. 8, 2024) —
“For the 850 mcg dose FDA claims is safe using the wrong types of aluminum, the wrong type of mammal, the wrong route of exposure, and a single cherry-picked, misinterpreted study, a person would have to weigh 374 pounds to avoid exceeding the FDA’s aluminum safe level for intravenous exposure.”
In 2019, the Informed Consent Action Network (ICAN) sent US HHS requested, via FOIA:
“Copies of any human or animal studies involving the subcutaneous or intramuscular injection of aluminum adjuvant relied upon by the CDC to establish the safety of injecting infants and children with aluminum hydroxide, aluminum phosphate or amorphous aluminum hydroxyphosphate sulfate.”
US HHS with the following:
“A search of our records failed to reveal any documents pertaining to your request. Specifically, CDC’s Immunization Safety Office (ISO) states that ‘[t]his request is outside of ISO purview and should be referred to the U.S. Food and Drug Administration.[FDA]’ FDA’s contact information is as follows:”
An appeal was filed, and ICAN was ultimately told by HHS to check the peer-reviewed literature:

HHS suggested they use “the following NIH webpages to possibly find publicly available information relevant to your request”. That request included Pubmed.
By the time HHS had replied, IPAK had already gone over the entire literature and published the full story of how 850 mcg of aluminum was determined that an 850 mcg dose of aluminum hydroxide per dose was “safe”.
We had provided the world with the first pediatric dose limit (PDL) of exposure to aluminum hydroxide in vaccines based on standard toxicological consideration of body weight. This is particularly important since the same compound is used to routinely and reliably induce autoimmune conditions in rats and mice at doses per body weight that overlap those human infants experience during early to mid-infancy. However, the FDA’s 850 mcg per dose was not based on body weight.
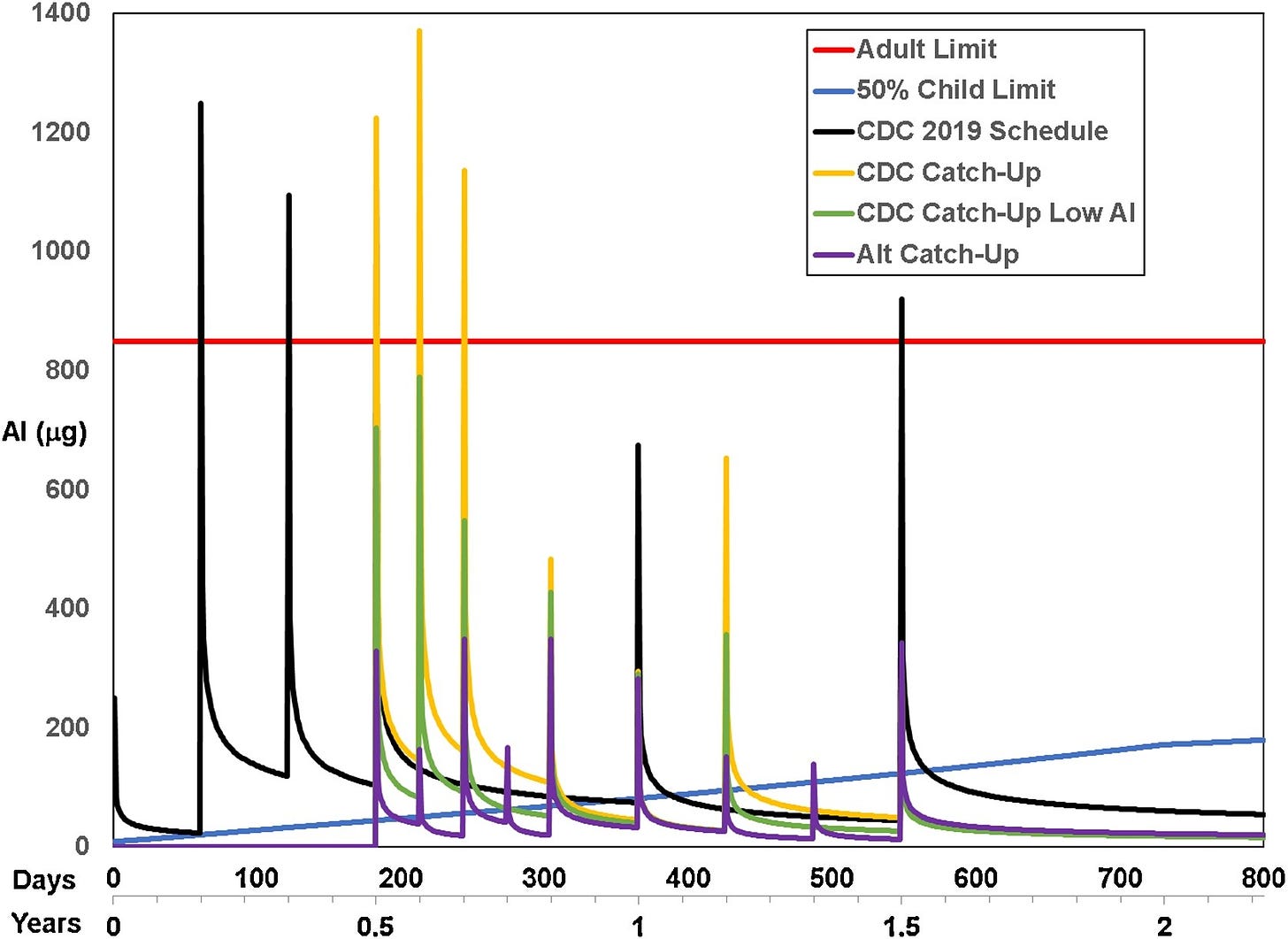
See the blue line in this image? That’s the body-weight adjusted PDL we published (also based on consideration to kidney development up to two years) of the 50%tile body weight of children at each age. The orange line is FDA’s 850 mcg so-called ‘safe’ dose of of 850 mcg per vaccine. The lines with the peaks correspond to uptake and clearance under various schedules for the various schedules studied.
Clearly, aluminum exposure does not only have acute toxicity (peaks); it also has chronic exposure – wherever the clearance lines exceed the blue PDL line, infants and toddlers are experiencing the health effects of whole-body aluminum toxicity, including risks of autoimmunity and food allergies.
Read the rest here.


